All product information is provided by the supplier. The National Board of Social Services is not responsible for either contents, origin, flaws and deficiencies, or any kind of damage that may occur from the use of the information. The National Board of Social Services has no authority to endorse products and does not assess the quality of the products. Hide this message.
SAFE Med trykaflastende Helmadras nr.201YP,GUL-ROSA, brug op til 180kg
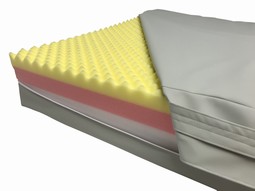
SAFE Med pressure relieving Mattress no. 201YP molds to the body for efficient pressure distribution and stability to prevent pressure sores and promote healing. The visco-elastic SAFE foam ensures a comfortable body temperature.
Incontinence cover is breathable and stretchable with covered zipper on three sides.
Choose 12 or 14 cm high mattress
Incontinence cover is breathable and stretchable with covered zipper on three sides.
Choose 12 or 14 cm high mattress
Classification
04 33 06 13 - Foam mattresses, synthetic (PUR)
The product series contains 6 products.
Product 1 of 6
SAFE Med pressure relieving Mattress no.201YP up to 130 kg,200x80x12cm
HMI-no.
81408
Article-no.
201YP.1
Registration date
23-10-2013
Last updated
21-11-2023
Properties
Intended for children
No

Products for children must comply with the specific demands for safety as stated in certain standards. It is the supplier of the product who has stated that the product is intended for children. The National Board of Social Services holds no responsibility in relation to this assessment.
HR PUR foam
Yes

High Resilience Polyurethane foam
CM HR PUR foam
No

Combustion Modified High Resilience foam
Viscoelastic PUR foam
Yes

Temperature sensitive polyurethane foam
Intended for use with adjustable mattress support platform
Yes
Measures
Length
200
cm

For mattress extension: measure of the elongation of the mattress.
Width
80
cm

For mattress extension: equivalent to the widht of the mattress.
Height
12
cm
Mattress weight
14
kg
User weight, max
130
kg
Test information 

Though the product is testet, it might not have passed all requirements in the standard. Besides, some products are only tested according to parts of the standard. Read the test report for detailed information. See also other standards that could be relevant for this product type.
: Tested according to other national or international standard.
Test lab: Swerea IVF AB. Test date: 07-11-2018 Test report for HMI-no. 81408
Test report for HMI-no. 81408
Test lab: Swerea IVF AB. Test date: 07-11-2018
 Test report for HMI-no. 81408
Test report for HMI-no. 81408EU product safety regulation 

The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 2 of 6
SAFE Med pressure relieving Mattress no.201YP,YELLOW-PINK,200x80x12cm
HMI-no.
114319
Article-no.
201YP.14.1
Registration date
28-01-2019
Last updated
21-11-2023
Properties
Intended for children
No

Products for children must comply with the specific demands for safety as stated in certain standards. It is the supplier of the product who has stated that the product is intended for children. The National Board of Social Services holds no responsibility in relation to this assessment.
HR PUR foam
Yes

High Resilience Polyurethane foam
CM HR PUR foam
No

Combustion Modified High Resilience foam
Viscoelastic PUR foam
Yes

Temperature sensitive polyurethane foam
Intended for use with adjustable mattress support platform
Yes
Measures
Length
200
cm

For mattress extension: measure of the elongation of the mattress.
Width
80
cm

For mattress extension: equivalent to the widht of the mattress.
Height
14
cm
Mattress weight
14.5
kg
User weight, max
180
kg
Test information 

Though the product is testet, it might not have passed all requirements in the standard. Besides, some products are only tested according to parts of the standard. Read the test report for detailed information. See also other standards that could be relevant for this product type.
: Tested according to other national or international standard.
Test lab: Swerea IVF AB. Test date: 07-11-2018 Test report for HMI-no. 114319
Test report for HMI-no. 114319
Test lab: Swerea IVF AB. Test date: 07-11-2018
 Test report for HMI-no. 114319
Test report for HMI-no. 114319EU product safety regulation 

The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 3 of 6
SAFE Med pressure relieving Mattress no.201YP,YELLOW-PINK,200x85x12 cm
HMI-no.
114322
Article-no.
201YP.14.20085
Registration date
28-01-2019
Last updated
21-11-2023
Properties
Intended for children
No

Products for children must comply with the specific demands for safety as stated in certain standards. It is the supplier of the product who has stated that the product is intended for children. The National Board of Social Services holds no responsibility in relation to this assessment.
HR PUR foam
Yes

High Resilience Polyurethane foam
CM HR PUR foam
No

Combustion Modified High Resilience foam
Viscoelastic PUR foam
Yes

Temperature sensitive polyurethane foam
Intended for use with adjustable mattress support platform
Yes
Measures
Length
200
cm

For mattress extension: measure of the elongation of the mattress.
Width
85
cm

For mattress extension: equivalent to the widht of the mattress.
Height
14
cm
Mattress weight
14.7
kg
User weight, max
180
kg
Test information 

Though the product is testet, it might not have passed all requirements in the standard. Besides, some products are only tested according to parts of the standard. Read the test report for detailed information. See also other standards that could be relevant for this product type.
: Tested according to other national or international standard.
Test lab: Swerea IVF AB. Test date: 07-11-2018 Test report for HMI-no. 114322
Test report for HMI-no. 114322
Test lab: Swerea IVF AB. Test date: 07-11-2018
 Test report for HMI-no. 114322
Test report for HMI-no. 114322EU product safety regulation 

The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 4 of 6
SAFE Med pressure relieving Mattress no.201YP,YELLOW-PINK,200x85x12 cm
HMI-no.
81409
Article-no.
201YP.20085
Registration date
23-10-2013
Last updated
21-11-2023
Properties
Intended for children
No

Products for children must comply with the specific demands for safety as stated in certain standards. It is the supplier of the product who has stated that the product is intended for children. The National Board of Social Services holds no responsibility in relation to this assessment.
HR PUR foam
Yes

High Resilience Polyurethane foam
CM HR PUR foam
No

Combustion Modified High Resilience foam
Viscoelastic PUR foam
Yes

Temperature sensitive polyurethane foam
Intended for use with adjustable mattress support platform
Yes
Measures
Length
200
cm

For mattress extension: measure of the elongation of the mattress.
Width
85
cm

For mattress extension: equivalent to the widht of the mattress.
Height
12
cm
Mattress weight
14
kg
User weight, max
130
kg
Test information 

Though the product is testet, it might not have passed all requirements in the standard. Besides, some products are only tested according to parts of the standard. Read the test report for detailed information. See also other standards that could be relevant for this product type.
: Tested according to other national or international standard.
Test lab: Swerea IVF AB. Test date: 07-11-2018 Test report for HMI-no. 81409
Test report for HMI-no. 81409
Test lab: Swerea IVF AB. Test date: 07-11-2018
 Test report for HMI-no. 81409
Test report for HMI-no. 81409EU product safety regulation 

The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 5 of 6
SAFE Med trykaflastende Helmadras nr.201YP,GUL-ROSA, 200 x 90 x 12 cm
HMI-no.
81410
Article-no.
201YP.2
Registration date
23-10-2013
Last updated
21-11-2023
Properties
Intended for children
No

Products for children must comply with the specific demands for safety as stated in certain standards. It is the supplier of the product who has stated that the product is intended for children. The National Board of Social Services holds no responsibility in relation to this assessment.
HR PUR foam
Yes

High Resilience Polyurethane foam
CM HR PUR foam
No

Combustion Modified High Resilience foam
Viscoelastic PUR foam
Yes

Temperature sensitive polyurethane foam
Intended for use with adjustable mattress support platform
Yes
Measures
Length
200
cm

For mattress extension: measure of the elongation of the mattress.
Width
90
cm

For mattress extension: equivalent to the widht of the mattress.
Height
12
cm
Mattress weight
14
kg
User weight, max
130
kg
Test information 

Though the product is testet, it might not have passed all requirements in the standard. Besides, some products are only tested according to parts of the standard. Read the test report for detailed information. See also other standards that could be relevant for this product type.
: Tested according to other national or international standard.
Test lab: Swerea IVF AB. Test date: 07-11-2018 Test report for HMI-no. 81410
Test report for HMI-no. 81410
Test lab: Swerea IVF AB. Test date: 07-11-2018
 Test report for HMI-no. 81410
Test report for HMI-no. 81410EU product safety regulation 

The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 6 of 6
SAFE Med trykaflastende Helmadras nr.201YP,GUL-ROSA, 200 x 90 x 14 cm
HMI-no.
114325
Article-no.
201YP.14.2
Registration date
28-01-2019
Last updated
21-11-2023
Properties
Intended for children
No

Products for children must comply with the specific demands for safety as stated in certain standards. It is the supplier of the product who has stated that the product is intended for children. The National Board of Social Services holds no responsibility in relation to this assessment.
HR PUR foam
Yes

High Resilience Polyurethane foam
CM HR PUR foam
No

Combustion Modified High Resilience foam
Viscoelastic PUR foam
Yes

Temperature sensitive polyurethane foam
Intended for use with adjustable mattress support platform
Yes
Measures
Length
200
cm

For mattress extension: measure of the elongation of the mattress.
Width
90
cm

For mattress extension: equivalent to the widht of the mattress.
Height
14
cm
Mattress weight
14.9
kg
User weight, max
180
kg
Test information 

Though the product is testet, it might not have passed all requirements in the standard. Besides, some products are only tested according to parts of the standard. Read the test report for detailed information. See also other standards that could be relevant for this product type.
: Tested according to other national or international standard.
Test lab: Swerea IVF AB. Test date: 07-11-2018 Test report for HMI-no. 114325
Test report for HMI-no. 114325
Test lab: Swerea IVF AB. Test date: 07-11-2018
 Test report for HMI-no. 114325
Test report for HMI-no. 114325EU product safety regulation 

The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device