All product information is provided by the supplier. The National Board of Social Services is not responsible for either contents, origin, flaws and deficiencies, or any kind of damage that may occur from the use of the information. The National Board of Social Services has no authority to endorse products and does not assess the quality of the products. Hide this message.
Pelvic Cradle
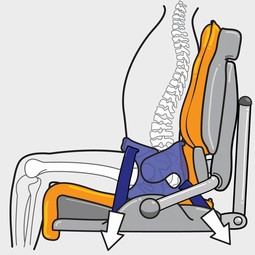
The flexible adjustment in the back section can encourage
a posteriorly tilted pelvis into neutral alignment, and the overall design prevents users sliding forward. This gives unsurpassed proximal
pelvic positioning and support for more complex body sha
a posteriorly tilted pelvis into neutral alignment, and the overall design prevents users sliding forward. This gives unsurpassed proximal
pelvic positioning and support for more complex body sha
Classification
09 07 03 01 - Belts without shoulder fixation
Documents
The product series contains 3 products.
Product 1 of 3
Pelvic Cradle, Size 0
HMI-no.
101702
Article-no.
840100100275
Registration date
26-05-2016
Last updated
21-11-2023
Properties
Intended for children
Yes

Products for children must comply with the specific demands for safety as stated in certain standards. It is the supplier of the product who has stated that the product is intended for children. The National Board of Social Services holds no responsibility in relation to this assessment.
Intended use in water
No
4-point fittings
Yes
Parts of the belt are between the legs
No
Secured from unintended unlocking
No
Measures
Length, min
62
cm

Measured between the anchorings.
Length, max
80
cm

Measured between the anchorings.
Test information
No information about tests according to standards 

The supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation 

The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 2 of 3
Pelvic Cradle, Size 00
HMI-no.
101701
Article-no.
840100100566
Registration date
26-05-2016
Last updated
21-11-2023
Properties
Intended for children
Yes

Products for children must comply with the specific demands for safety as stated in certain standards. It is the supplier of the product who has stated that the product is intended for children. The National Board of Social Services holds no responsibility in relation to this assessment.
Intended use in water
No
4-point fittings
Yes
Parts of the belt are between the legs
No
Secured from unintended unlocking
No
Measures
Length, min
48
cm

Measured between the anchorings.
Length, max
62
cm

Measured between the anchorings.
Test information
No information about tests according to standards 

The supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation 

The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 3 of 3
Pelvic Cradle, Size 1
HMI-no.
101703
Article-no.
840100100276
Registration date
26-05-2016
Last updated
21-11-2023
Properties
Intended for children
Yes

Products for children must comply with the specific demands for safety as stated in certain standards. It is the supplier of the product who has stated that the product is intended for children. The National Board of Social Services holds no responsibility in relation to this assessment.
Intended use in water
No
4-point fittings
Yes
Parts of the belt are between the legs
No
Secured from unintended unlocking
No
Measures
Length, min
64
cm

Measured between the anchorings.
Length, max
82
cm

Measured between the anchorings.
Test information
No information about tests according to standards 

The supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation 

The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device




 Brochure
Brochure