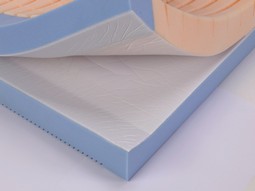
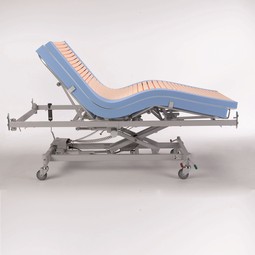
Softform Premier Maxi Glide
The mattress manages shear forces excellently when the bed is adjusted. As the two-layer design allows the lower layer to stay on the bed while the upper layer moves with the user, shear and friction are minimized and comfort is increased.
Classification
04 33 06 13 - Foam mattresses, synthetic (PUR)
Documents
The product serie includes 5 products. 3 of these products are discontinued from the supplier.
Product 1 of 5
SOFTFORM PREMIER MAXI GLIDE 83 200 15 CM, SRT COVER, WELDED SEAMS,
HMI-no.
147209
Article-no.
60136342
Registration date
28-01-2026
Last updated
29-01-2026
Properties
Intended for use with adjustable mattress support platform
Yes
Fire-retardant foam
No
Measures
Length
200
cm
Width
83
cm
Height
15
cm
Mattress weight
13
kg
User weight, max
247
kg
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 147209
EU declaration of conformity for HMI-no. 147209Product 2 of 5
Softform Premier Maxi Glide 83x200x14 cm
The product was discontinued from Invacare A/S 28-01-2026HMI-no.
47560
Article-no.
1514449
Registration date
21-01-2008
Last updated
29-01-2026
Properties
Intended for use with adjustable mattress support platform
Yes
Fire-retardant foam
No
Measures
Length
200
cm
Width
83
cm
Height
14
cm
Mattress weight
13
kg
User weight, max
247
kg
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 3 of 5
Softform Premier Maxi Glide 85x200x 14 cm
The product was discontinued from Invacare A/S 28-01-2026HMI-no.
139237
Article-no.
1534012
Registration date
24-11-2023
Last updated
29-01-2026
Properties
Intended for use with adjustable mattress support platform
Yes
Fire-retardant foam
No
Measures
Length
200
cm
Width
85
cm
Height
14
cm
Mattress weight
13
kg
User weight, max
247
kg
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 4 of 5
SOFTFORM PREMIER MAXI GLIDE 88 200 15 CM, SRT COVER, WELDED SEAMS,
HMI-no.
147210
Article-no.
60134389
Registration date
28-01-2026
Last updated
29-01-2026
Properties
Intended for use with adjustable mattress support platform
Yes
Fire-retardant foam
No
Measures
Length
200
cm
Width
88
cm
Height
15
cm
Mattress weight
13
kg
User weight, max
247
kg
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 147210
EU declaration of conformity for HMI-no. 147210Product 5 of 5
Softform Premier Maxi Glide 88x200x14 cm
The product was discontinued from Invacare A/S 28-01-2026HMI-no.
47561
Article-no.
1514478
Registration date
21-01-2008
Last updated
29-01-2026
Properties
Intended for use with adjustable mattress support platform
Yes
Fire-retardant foam
No
Measures
Length
200
cm
Width
88
cm
Height
14
cm
Mattress weight
13
kg
User weight, max
247
kg
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device