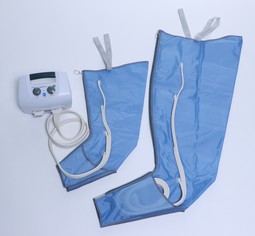
Apodan Lympha Press Benmanchet, 4 luftkamre
Classification
04 06 09 04 - Air-filled attachments and pulsating compression systems, leg
Documents
The product serie includes 20 products. 10 of these products are discontinued from the supplier.
Product 1 of 20
Lympha Press Benmanchet, 4 luftkamre, str. 2-50
HMI-no.
107380
Article-no.
024050
Registration date
27-11-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 2 of 20
Lympha Press Benmanchet, 4 luftkamre, str. 2-75
HMI-no.
109947
Article-no.
024045
Registration date
14-06-2018
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 3 of 20
Lympha Press Benmanchet, 4 luftkamre, str. 2-85
HMI-no.
113560
Article-no.
024044
Registration date
18-12-2018
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 4 of 20
Lympha Press Benmanchet, 4 luftkamre, str. 2-95
HMI-no.
113561
Article-no.
024043
Registration date
18-12-2018
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 5 of 20
Lympha Press Benmanchet, 4 luftkamre, str. 3-65
HMI-no.
107381
Article-no.
024051
Registration date
27-11-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 6 of 20
Lympha Press Benmanchet, 4 luftkamre, str. 3-75
HMI-no.
106944
Article-no.
024052
Registration date
13-11-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 7 of 20
Lympha Press Benmanchet, 4 luftkamre, str. 3-85
HMI-no.
107346
Article-no.
024053
Registration date
23-11-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 8 of 20
Lympha Press Benmanchet, 4 luftkamre, str. 4-65
HMI-no.
107382
Article-no.
024046
Registration date
27-11-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 9 of 20
Lympha Press Benmanchet, 4 luftkamre, str. 4-75
HMI-no.
107383
Article-no.
024047
Registration date
27-11-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 10 of 20
Lympha Press Benmanchet, 4 luftkamre, str. 4-85
HMI-no.
107384
Article-no.
024048
Registration date
27-11-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 11 of 20
Phlebo Press Benmanchet, 4 luftkamre, str. 2-50
The product was discontinued from Apodan A/S 12-06-2019HMI-no.
65097
Article-no.
024008
Registration date
12-09-2011
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 12 of 20
Phlebo Press Benmanchet, 4 luftkamre, str. 2-75
The product was discontinued from Apodan A/S 12-06-2019HMI-no.
65098
Article-no.
024001
Registration date
12-09-2011
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 13 of 20
Phlebo Press Benmanchet, 4 luftkamre, str. 2-85
The product was discontinued from Apodan A/S 14-06-2018HMI-no.
65111
Article-no.
024002
Registration date
12-09-2011
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 14 of 20
Phlebo Press Benmanchet, 4 luftkamre, str. 2-95
The product was discontinued from Apodan A/S 14-06-2018HMI-no.
65099
Article-no.
024004
Registration date
12-09-2011
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 15 of 20
Phlebo Press Benmanchet, 4 luftkamre, str. 3-65
The product was discontinued from Apodan A/S 12-06-2019HMI-no.
65110
Article-no.
024006
Registration date
12-09-2011
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 16 of 20
Phlebo Press Benmanchet, 4 luftkamre, str. 3-75
The product was discontinued from Apodan A/S 16-11-2017HMI-no.
65100
Article-no.
024007
Registration date
12-09-2011
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 17 of 20
Phlebo Press Benmanchet, 4 luftkamre, str. 3-85
The product was discontinued from Apodan A/S 23-11-2017HMI-no.
65101
Article-no.
024003
Registration date
12-09-2011
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 18 of 20
Phlebo Press Benmanchet, 4 luftkamre, str. 4-65
The product was discontinued from Apodan A/S 14-06-2018HMI-no.
106526
Article-no.
024046
Registration date
25-09-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 19 of 20
Phlebo Press Benmanchet, 4 luftkamre, str. 4-75
The product was discontinued from Apodan A/S 14-06-2018HMI-no.
106527
Article-no.
024047
Registration date
25-09-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 20 of 20
Phlebo Press Benmanchet, 4 luftkamre, str. 4-85
The product was discontinued from Apodan A/S 14-06-2018HMI-no.
106528
Article-no.
024048
Registration date
25-09-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device






 Brochure
Brochure