BioWaveGO
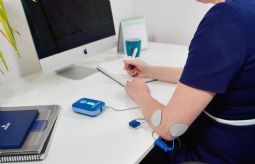
BioWaveGO, uses high-frequency neurostimulation through the skin directly to the pain nerves, inhibits the propagation of the action potential and blocks the transmission of pain signals to the brain.
100 pct. drug-free, wearable, FDA-cleared and CE-certified, smarter pain-blocking technology.
Easy to use and long-term effect on 8h after 30min use.
100 pct. drug-free, wearable, FDA-cleared and CE-certified, smarter pain-blocking technology.
Easy to use and long-term effect on 8h after 30min use.
Classification
04 29 00 01 - Assistive products for pain relief
Documents
The product series contains 1 product.
Product 1 of 1
BioWaveGO
HMI-no.
136235
Article-no.
BWG
Registration date
24-03-2023
Last updated
04-05-2023
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device






 Brochure
Brochure