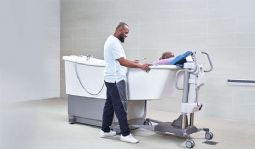
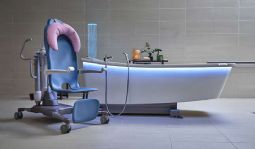
Symbliss Bath
The Symbliss bathtub is part of an integrated bathing system for care recipients at all levels of mobility. Its design enables care staff to deliver a hygiene solution efficiently and safely, supporting healthier outcomes. A wellness break that provides comfort for body and mind.
Classification
09 33 21 03 - Bathtubs
Documents
The product series contains 2 products.
Product 1 of 2
Symbliss Bath with footrest
HMI-no.
144511
Article-no.
SBS070EU01
Registration date
15-05-2025
Last updated
16-05-2025
Properties
Bathtub
Yes
Movable
No
Measures
External width
90
cm
External length
211
cm
Height, min
80
cm
Height, max
118
cm
User weight, max
272
kg
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 144511
EU declaration of conformity for HMI-no. 144511Product 2 of 2
Symbliss Bath without footrest
HMI-no.
144512
Article-no.
SBS070EU00
Registration date
15-05-2025
Last updated
16-05-2025
Properties
Bathtub
Yes
Movable
No
Measures
External width
90
cm
External length
211
cm
Height, min
80
cm
Height, max
118
cm
User weight, max
272
kg
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 144512
EU declaration of conformity for HMI-no. 144512