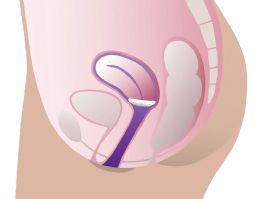
ProFem Shaatz Vaginal pessar
Relieves discomfort caused by cystocele and second- or third-degree uterine prolapse. The curved shape ensures a comfortable fit, while the holes prevent vacuum and ease removal. Made from hypoallergenic medical-grade silicone. Initial use should begin with a fitting set.
Classification
09 31 03 01 - Vaginal devices for compression of the urethra
Documents
The product series contains 9 products.
Product 1 of 9
ProFem Shaatz Pessary - size
HMI-no.
145655
Article-no.
SH2.00-2
Registration date
02-09-2025
Last updated
15-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145655
EU declaration of conformity for HMI-no. 145655Product 2 of 9
ProFem Shaatz Pessary - size 0
HMI-no.
145649
Article-no.
SH1.50-0
Registration date
02-09-2025
Last updated
15-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145649
EU declaration of conformity for HMI-no. 145649Product 3 of 9
ProFem Shaatz Pessary - size 1
HMI-no.
145652
Article-no.
SH1.75-0
Registration date
02-09-2025
Last updated
15-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145652
EU declaration of conformity for HMI-no. 145652Product 4 of 9
ProFem Shaatz Pessary - size 3
HMI-no.
145658
Article-no.
SH2.25-3
Registration date
02-09-2025
Last updated
15-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145658
EU declaration of conformity for HMI-no. 145658Product 5 of 9
ProFem Shaatz Pessary - size 4
HMI-no.
145661
Article-no.
SH2.50-4
Registration date
02-09-2025
Last updated
15-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145661
EU declaration of conformity for HMI-no. 145661Product 6 of 9
ProFem Shaatz Pessary - size 5
HMI-no.
145664
Article-no.
SH2.75-5
Registration date
02-09-2025
Last updated
15-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145664
EU declaration of conformity for HMI-no. 145664Product 7 of 9
ProFem Shaatz Pessary - size 6
HMI-no.
145667
Article-no.
SH3.00-6
Registration date
02-09-2025
Last updated
15-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145667
EU declaration of conformity for HMI-no. 145667Product 8 of 9
ProFem Shaatz Pessary - size 7
HMI-no.
145670
Article-no.
SH3.25-7
Registration date
02-09-2025
Last updated
15-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145670
EU declaration of conformity for HMI-no. 145670Product 9 of 9
ProFem Shaatz Pessary - size 8
HMI-no.
145673
Article-no.
SH3.50-8
Registration date
02-09-2025
Last updated
15-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145673
EU declaration of conformity for HMI-no. 145673